AWARDEES: Craig T. Clark, Lourdes J. Cruz, J. Michael McIntosh, and Baldomero Marquez Olivera
FEDERAL FUNDING AGENCIES: Department of Defense, National Institutes of Health
When Baldomero “Toto” Olivera and Lourdes “Luly” Cruz started studying cone snails in the 1970s, they didn’t know that their work would change the course of their careers. They didn’t know they were embarking on a decade- and globe-spanning partnership, or that with the help of two undergraduates—Craig Clark and Michael McIntosh—they would discover the raw material for a non-opioid pain reliever and a powerful new tool for studying the central nervous system, all hidden in the cone snail’s potent venom.
They were just trying to solve a supply chain problem.
Becoming Scientists
Lourdes Cruz (Lourdes Cruz)
Lourdes Cruz was perhaps always destined to be a scientist. The daughter of a chemist and a dentist, whose older sister was also a chemist, she attended the University of the Philippines, where she met, but never had a class with, Baldomero Olivera. Like many of her classmates, Cruz then attended graduate school in the United States before returning to the Philippines. Eventually, after a stint at a research institute focused on rice, she found her way back to the University of the Philippines, where she began working with her former classmate, Olivera. Affectionally called “Toto” by his colleagues, Olivera grew up in the Philippines and San Francisco, where his father served as a press attaché for the Philippine consulate. It was in San Francisco, in second grade, that he met the first of many influential teachers who would push him toward his eventual career.
“There was a teacher in second grade—Miss Uhler, I still remember her name—she did a scientific experiment, and after that, I was hooked,” Olivera says. “I never wanted to be anything else but a scientist.”
Baldomero “Toto” Olivera
Now in his 80s, Olivera still remembers many of his early teachers by name. In high school Olivera took up shell collecting, an easy hobby to reach for in a country of over 7,000 islands. Another of his teachers encouraged him to take a more scientific approach to his hobby. He did not need much persuading. From the very beginning, Olivera was interested in cone snails. He even wanted to study the animals for his undergraduate thesis, but his advisory committee dissuaded him. “They said you won’t be able to do anything with that,” Olivera recalls.
Fast forward about a decade to the early 1970s. Cruz has just joined the Biochemistry Department. Olivera is spending part of each year in the Philippines while also working on DNA synthesis at Kansas State University (as a matter of fact, Olivera was one of the researchers who discovered DNA ligase, a key enzyme used for DNA replication and repair). But DNA research in the Philippines was impeded by a lack of equipment and bureaucratic barriers to accessing supplies. Cruz says the supply issues were consistent enough to spawn a running joke: “‘By the time it comes, you’ve forgotten why you bought it!’ So we thought, let’s think of a project where we will have an advantage.”
Olivera remembered the colorful snail shells that had fascinated him as a child. Cone snails are abundant in the Philippines, and they could start researching the nature of their venom with limited equipment. It seemed like a worthwhile side project.
There are as many as a thousand different species of cone snails (University of Utah)
The Shell Collectors
The umbrella term “cone snail” refers to a massive variety of venomous animals—up to as many as a thousand different species. Named for their conical shells and prized by shell collectors, cone snails hunt in a variety of ways and have specialized diets. Some cone snails eat worms, some eat other mollusks, some eat fish. But how does such a slow-moving animal catch something as fast as a fish? One common strategy involves a snail burying itself in the sand and extending its proboscis, much like a fishing line. When the tip of the proboscis touches fish skin, the snail ejects a hypodermic needle-like structure from the end of the line, harpooning the fish and allowing paralytic venom to flow through the hollow proboscis. Then the snail reels in the fish and swallows it whole.
Olivera knew much of this from his shell collecting days. More importantly, he knew shell collectors, and getting to know them became a big part of the job: “During lunchtime, we would go visit the shops and talk to the shell dealers,” Cruz says. “It was fun. They would show us everything they had and connect us with suppliers.”
Although cone snails, like other mollusks with beautiful shells, are still threatened by the global shell trade, the industry is somewhat more regulated now than it was in the 1970s. Cruz recalls being amazed at how many shells were being collected at the time. It was a big business and required some tricky negotiation. For the rarest and most expensive species, the team would essentially borrow the animal for a while before returning the shell to the supplier to be sold. The lab was within walking distance of Manila Bay, and Cruz recalls walking to the beach to collect other snails for the snail-eating cones.
Some cone snails, especially some of the larger species, pose a threat to humans. One species of great importance to Olivera’s lab, Conus geographus, is also commonly known as the cigarette cone, reputedly because if a person is stung by it, they will only have time to smoke a single cigarette before they die. When asked how the team handled such dangerous creatures, Cruz suggests they are quite shy, more inclined to retreat into their shells than to sting you. “People who have been stung and died are generally people who have held them for a long time,” she suggests, recalling once interviewing a spear fisher who saw a snail while pursuing fish. Hoping to capture both, he put the snail under the elastic of his trunk and was stung when the snail emerged. He lived to tell the tale, but only after a three-week hospital stay. “We were very careful!” Cruz says.
A cone snail eats a paralyzed fish (University of Utah)
Bit by bit, the team got to know the mysterious animals. The snails are nocturnal, and the team would stay up late just watching them. “We gave them names,” Cruz recalls, “and you could see that they had different personalities.” Although there were certainly bumps: one snail, for example, went from Gerald to Geraldine when she turned out to be female. A bigger hiccup came from putting the wrong species in a tank together, leading to lots of full mollusk-eating cone snails and lots of empty shells. “After that, we put the mollusk-eaters in separate aquaria,” Cruz says with a laugh.
One early discovery concerned the nature of the venom itself. At the time, the researchers did not even know if the toxin was a carbohydrate or a protein. Earlier work by other researchers had functionally ruled out a protein because the compound did not appear to be digested by protease, a kind of enzyme that breaks down proteins. But the Manila lab tested the venom in different conditions, including a longer incubation period, and that worked. What explains the difference?
“We realized that most of the bioactive ingredients in the venom are very small proteins—peptides,” Olivera says. “In fact, they’re unusually small—that was one of the surprises.” While the bioactive component in snake venom is about 50 amino acids in length, the first components of the purified cone snail venom were only about 13 amino acids in length. Moreover, they were very tightly bound to their target, which explains why they were so difficult for the protease to digest.
Discovering that the venom’s bioactive compounds were peptides was the first of many “ah ha!” moments in the duo’s research. The methods for isolating protein have been very well-developed, and the team was able to rapidly learn a lot about the protein’s various components. Unfortunately, progress on the project was complicated by the imposition of martial law in the Philippines. Olivera changed jobs, and he and his family settled in Utah while Cruz stayed in Manila. She recalls being in the lab and listening to the radio fuzz out on the night martial law was declared. Still, the two could collaborate at a distance, and several years later, Cruz got a position alongside Olivera at the University of Utah. Now it was her turn to go back and forth.
Although it sometimes led to logistical difficulties, the multi-national nature of the research was a huge part of its success. Thanks to their access to cone snails, the lab in the Philippines was able to provide a tremendous amount of crude venom to work with, while the Utah lab gave them access to leading-edge technology to analyze that material. “I would say, in retrospect, that’s what allowed us to move faster, given the constraints,” says Olivera.
An injection of funding from the National Institute of General Medical Sciences (NIGMS), part of the National Institutes of Health (NIH), further catalyzed the project by allowing the team to access more sophisticated equipment. “It wasn’t that easy to analyze small amounts of proteins at the time,” says Olivera. “But the fact that we finally got an NIH grant, that I would say was what really allowed us to discover things that were unexpected.”
That, plus the timely interventions of two undergraduate students.
Craig Clark (Lisa Roa)
A Breakthrough
Cruz recalls Craig Clark, an undergraduate student in biology who worked in the lab, as being particularly curious about the brain. That curiosity led him to discover the technique that changed the course of their research. At the time, the team injected different fractions—that is, different combinations of components—of the venom into the skin of lab mice. But they were not finding anything remarkable. Then Clark had a different idea.
Craig wanted to see what would happen if he injected the venom into the brain, but Olivera was not so sure. “I was skeptical,” he recalls, “I really was.” But Clark was determined, and Olivera and Cruz encouraged experimentation in the lab. After learning how to do cranial injections from colleagues at the college of medicine, Clark ran his first experiment. The results were astonishing.
Right away, the team observed a huge range of dramatically different, sometimes even opposing, behavioral effects in the mice. Once injected, some fractions of the toxin caused hyper-excitability, while others made mice lethargic. Some caused shaking, scratching, or falling over. The results were so unexpected and specific that the team knew right away that something special was happening.
“When Craig developed that assay, and we saw these mice doing all these different things, I think that’s the moment when we realized this wasn’t just a bunch of paralytic toxins,” Olivera recalls. “There’s something much more complicated and wonderful here.” The team’s working hypothesis was that each venom component was targeting highly specific areas in the brain, either activating or inhibiting the messages they carried. But understanding that mechanism was a gradual process, and one particular venom fraction held the key.
J. Michael McIntosh
Tiny Snail, Big Impact
J. Michael McIntosh joined the team at just 18 years old, about six months after the lab started using Clark’s brain injection technique. “Michael [McIntosh] is a very methodical guy,” Olivera recalls, “And he wanted to purify a very specific thing.” That thing? The “shaker” peptide, or the fraction of venom that made mice shake. Over time, working shoulder-to-shoulder with Cruz, Clark, and Olivera, McIntosh purified the peptide and identified its chemical structure. They called it the omega-conotoxin.
Over the next few decades, and in partnership with many other collaborators, the team learned more about the omega-conotoxin. One of their first and most important discoveries was that it is paralytic to fish and frogs but not to mice. To understand why that’s a big deal, it helps to know how the body carries signals to the brain.
Lourdes Cruz working in the University of Utah lab (Lourdes Cruz)
Imagine you want to move your little finger. Your brain sends a signal—move my legs, please, Something is trying to eat you!—along a nerve, which then transfers to the muscle with the help of message-delivery devices called calcium channels. By working with frogs, the team and their collaborators proved that the omega-conotoxin was blocking those calcium channels, which meant the message—move your legs, please!—was not getting through. So why wasn’t the omega-conotoxin paralyzing the mice?
The answer is a trick of evolution. It turns out that we mammals have the same calcium channel that the omega-conotoxin can use to cause paralysis in frogs, but we use it differently. Whereas frogs and fish use it to control muscle movement, we use it to feel pain. Over several decades, this realization led to the development of the omega-conotoxin into a potent pain reliever, ziconotide, commercially known as Prialt. Interestingly, unlike most drugs derived from animal products, which accrue enhancements and modifications on their way to human medicine, ziconotide is a perfect synthetic copy of the omega-conotoxin, nothing added or taken away.
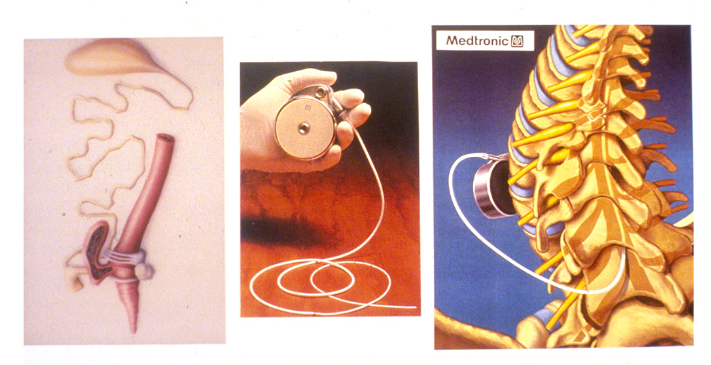
Patients who take Prialt are equipped with a programmable and refillable pump, about the size of a hockey puck, which is implanted into the abdominal cavity and only needs to be refilled every few months. While this necessarily limits the medication’s reach, it can be used as an alternative treatment to many more common opioid pain relievers. This alternative has been life-changing for the patients who use it, many of whom are battling cancer, AIDs, or other chronic conditions and have been unable to manage severe pain in any other way. It is a stunning, thoroughly unexpected result from what McIntosh called “a discovery project.” “We weren’t aiming to develop a pain compound,” he says.
The Prialt pump (center and right photos) operates in a very similar way to the pump the cone snail uses to paralyze fish (left photo) (University of Utah)
But that’s not the end of the story. Because of their ability to cause such specific behavioral effects in pre-clinical models, conotoxins have helped researchers develop new ways to map the body’s nervous system. When McIntosh discovered the omega-conotoxin, calcium channels were not very well understood. As Olivera points out, the lab’s insights regarding conotoxins launched a major initiative in neuroscience. Using conotoxins as probes, some neuroscientists are mapping the brain’s calcium channels and learning ever more about what they do. Other scientists are exploring the possibility of using conotoxins to treat a surprisingly wide range of illnesses, including addictions, epilepsy and diabetes.
McIntosh and Olivera in the office
Today, Olivera and McIntosh are still working together. After leaving the University of Utah to study medicine and psychiatry, McIntosh found himself continually drawn back to the cone snail research. Eventually, he found his way back to the lab permanently. He now works alongside Olivera in addition to serving as the medical director of Primary Care Mental Health Integration at Salt Lake City’s veteran’s hospital.
Cruz receiving the title of National Scientist of the Philippines from President Gloria Arroyo in 2006
Among other honors, Cruz is the recipient of the L'Oréal-UNESCO For Women in Science Awards. In 2006, she was named a National Scientists of the Philippines, the highest award accorded to Filipino scientists by the Philippine government. And though she has now retired from lab work, Cruz remains a tireless advocate for sustainability and community. She currently leads the Future Earth Philippines Program (FEPP), a scientific endeavor aimed at strengthening the country’s resilience and national sustainability as part of the global Future Earth network.
Tragically, Craig Clark passed away suddenly in 1994. But his wild idea to inject venom into the brain is still paying dividends in medicine and neuroscience, and all the members of the team consider that eureka moment as one of the most important in a project that was filled with unexpected flashes of serendipity and innovation.
By Haylie Swenson